Scientists have developed a revolutionary catalyst that not only converts CO2 into valuable products but actually increases in activity over time.

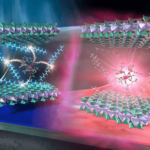
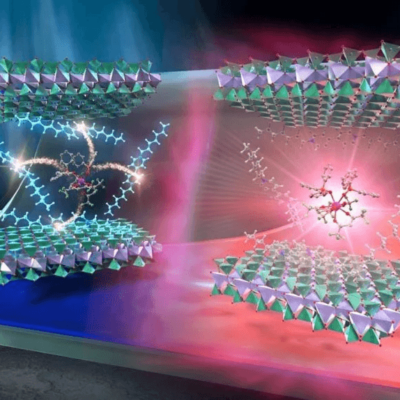
Made from tin microparticles on a nanotextured carbon structure, this innovative electrocatalyst efficiently produces formate—a key compound for various industries. Unlike conventional catalysts that degrade, this one self-optimizes by breaking down into smaller tin nanoparticles, dramatically improving performance.
Breakthrough Catalyst for CO2 Conversion
Scientists have developed a sustainable catalyst that becomes more effective as it operates, converting carbon dioxide (CO2) into valuable products. This breakthrough provides a foundation for designing next-generation electrocatalysts.
A research team from the University of Nottingham’s School of Chemistry and the University of Birmingham created the catalyst using tin microparticles supported by a nanotextured carbon structure. The interaction between the tin particles and graphitized carbon nanofibers plays a crucial role in transferring electrons from the carbon electrode to CO2 molecules—an essential step in converting CO2 into formate when an electric potential is applied.
These findings were published today (February 10) in ACS Applied Energy Materials, a journal of the American Chemical Society that focuses on interdisciplinary research in materials for energy applications.
Addressing CO2 Emissions with Electrocatalysis
CO2 is the primary contributor to global warming. While CO2 can be converted into useful products, traditional thermal methods typically rely on hydrogen sourced from fossil fuels. Therefore, it is essential to develop alternative methods like electrocatalysis, which utilizes sustainable energy sources, such as photovoltaics and wind power, as well as the abundant availability of water as a hydrogen source.
In electrocatalysis, applying an electric potential to the catalyst drives electrons through the material to react with CO2 and water, producing valuable compounds. One such product, formate, is widely used in the chemical synthesis of polymers, pharmaceuticals, adhesives, and more. For optimal efficiency, this process must operate at low potential while maintaining high current density and selectivity, ensuring effective use of electrons to convert CO2 to desired products.
Nanotextured Carbon Enhances Catalyst Performance
Dr. Madasamy Thangamuthu, a research fellow at the University of Nottingham co-led the research team, he said: “A successful electrocatalyst must strongly bond to the CO2 molecule and efficiently inject electrons to break its chemical bonds. We developed a new type of carbon electrode that incorporates graphitized nanofibers with a nanoscale texture, featuring curved surfaces and step edges, to enhance interaction with tin particles.”
Tom Burwell, a research assistant at the University of Nottingham undertook the work whilst studying at Centre for Doctorial Training in Sustainable Chemistry. He developed the approach and carried out the experimental work, he said: “We can assess the performance of the catalyst by measuring the electrical current consumed by the reacting CO2 molecules. Typically, catalysts degrade during use, resulting in decreased activity. Surprisingly, we observed the current flowing through tin on nanotextured carbon increased continuously over 48 hours. Analysis of the reaction products confirmed nearly all electrons were utilized to reduce CO2 to formate, boosting productivity by a factor of 3.6 while maintaining nearly 100% selectivity.”